Making research access and new care options happen anywhere in the U.S
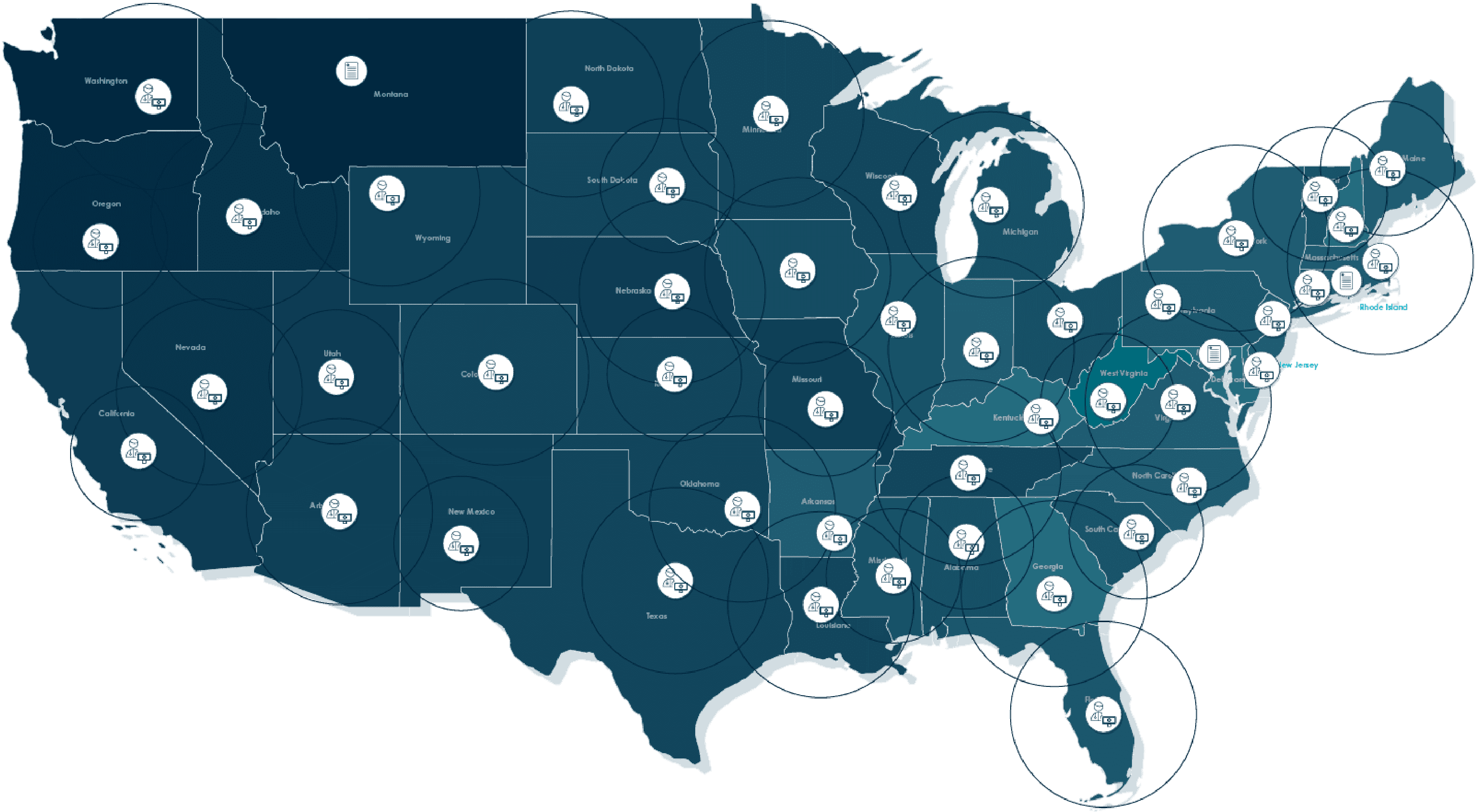
The Remote Site Network is built to support decentralized clinical trial models and comprises PIs and experienced research staff with coverage across the entire United States. This flexibility allows sponsors to provide greater access and inclusion to patients, meeting them where they are.
Remote Site Network
The Remote Site Network, part of our Community Research Network, is a national network of clinical trial investigators and experienced research staff, who are equipped to deliver on decentralized clinical trials. This flexibility allows sponsors to provide greater access and inclusion to patients, meeting them where they are.
Key Trial Attributes for the Remote Site Network
- Intervention: Observational/RWE
- Disease state: Well characterized
- Approval stage: Supplemental filling
- Few tele-visits and home health visits
- Short study duration
- Established registry
Example studies
Oncology | Screening Protocol
Active—Universal screening protocol with buccal swab for multiple oncology indications
Cardio-Metabolic | Screening Protocol
Start-up—Universal screening protocol for diabetes, obesity, cardiometabolic disorders
Features
Testimonials
I am very excited to be working with the team at Circuit Clinical. Clinical research has added a new dimension to my medical practice…
I am inspired by this field of medicine, and I feel very fortunate to be working with a cohesive team.
Remote Site Network
Principal Investigator

Featured Case Study
Ongoing Type 1 Diabetes Registry Achieving Success
The Remote Site Network is building a patient registry for an ongoing randomized controlled trial to demonstrate the efficacy of an investigational product compared to multiple daily injections for treatment of Type 1 diabetes.
Featured Case Study
Smoking Cessation Observational Study
An observational study consisting of three telehealth visits spanning over 30 states with 19 remote Principal Investigators. The first visit reviewed study requirements, confirm patient ID, and push ICF to patient via platform. The second visit reviewed eligibility, notify patient of study group assignment, and push questionnaire to patient. The final visit collected adverse event information as necessary.

Want to learn more about our Remote Site Network?
Contact our team to discuss our solutions for clinical research.
Learn more about how we support traditional trials with our Site Partnership Network, part of our Community Research Network.
Site Partnership Network